BLADDER CANCER

Bladder cancer consists of the neoplastic transformation of the cells lining the internal surface of
the bladder. The most common form of bladder cancer, named urothelial, is a tumor that begins
in the urothelial cells, which line the urethra, bladder, ureters, renal pelvis, and some other
organs. The majority (>90% 1) of bladder cancers are urothelial carcinomas.
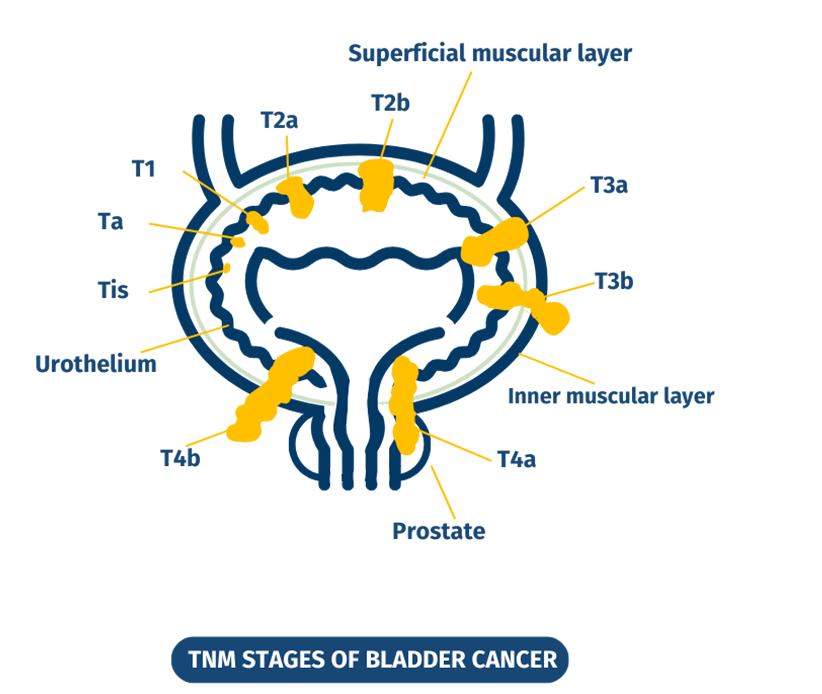
Bladder tumors are distinguished according to
the depth they reach and whether they are
limited to the internal surface of the bladder
(superficial or non-invasive tumors - NMIBC,
diagnosed in approximately 85% of cases), or
whether they have invaded the muscular wall
of the bladder (muscle invasive tumors - MIBC,
which represent approximately 15% of cases).
EPIDEMIOLOGY OF BLADDER CANCER

Bladder cancer represents approximately 3% of all
cancers, affecting over 313,500 individuals. An
incidence of around 30,000 cases/year is
estimated, with onset prevalent in old age,
between 60 and 70 years old.
In men, bladder cancer is 3 times more frequent
than in women and represents the fourth most
common neoplasm in terms of incidence and
alone accounts for 9% of tumors affecting men
aged between 50 and 69, a percentage which rises
to 11% of tumors in the age group aged over 70
years.
The probability of recurrence, i.e. the reappearance
of the tumor after some time, is very frequent and
has been estimated between 50% and 80 ¹.
1. AIOM-AIRTUM-SIAPEC-IAP. I numeri del cancro in Italia, Ottobre 2020

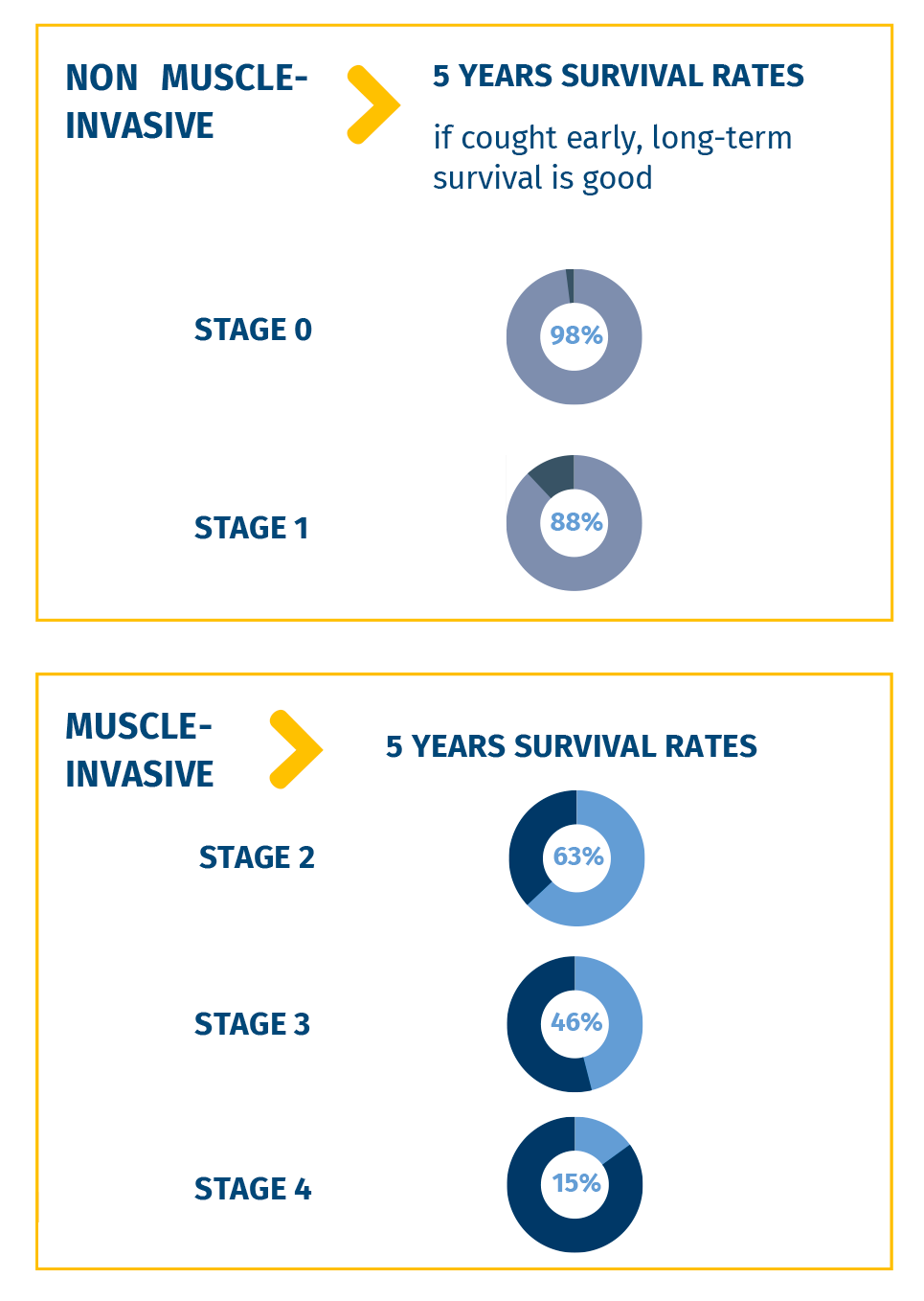
THE IMPORTANCE OF EARLY DIAGNOSIS

An early and accurate diagnosis of bladder cancer is
essential for the effectiveness of treatment, because
it can broaden the spectrum of therapeutic options
available and therefore increase the chances of
recovery.
When an early diagnosis is achieved, especially in
the early stages of tumor development, before it has
invaded the muscular wall of the bladder, the longterm
survival rate is very high.
In general, 5-year survival is approximately 80% for
both men and women, which must be interpreted in
light of the large prevalence of superficial forms
(with better prognosis) compared to those infiltrating the
muscle wall, for which the survival rate is drastically
reduced.
There is also a strong gradient by age: 96% in >45
years which reduces to 66% in 75+ years².
2. AIRTUM Working Group (2017). La sopravvivenza dei pazienti oncologici in Italia.
DIAGNOSIS OF BLADDER CANCER


- Diagnosis of urothelial carcinomas
- Follow-up of high-grade bladder
- Tumors Low cost
- Non-invasive


- Diagnosis of urothelial carcinomas
- Follow-up of high-grade bladder
- Tumors Low cost
- Non-invasive


- Expensive
- Invasive
- Does not detect all lesions³
- Operator dependent
- Low patient compliance

The scientific community showed the need for a non-invasive and highly accurate test to improve the management of patients with bladder cancer by reducing the number of cystoscopies required for triage of patients with hematuria and for monitoring patients with bladder cancer.
3. Yafi et al. 2015. UrolOncol 2015;33, 66 e25-31.
4. W. Devlies, J.J. de Jong, F. Hofmann et al., (....) A Systematic Review from the European Association of Urology Guidelines Office, Eur Urol Focus (2023)

AN ADVANCED GENETIC TEST FOR EARLY DIAGNOSIS OF BLADDER CANCER

UROADVANCE is an advanced genetic test that allows the non-invasive identification of somatic mutations
of urinary tumor DNA (utDNA) deriving from urothelial cells, associated with bladder cancer, through the
analysis of a simple urine sample.
The UROADVANCE test allows for early identification of bladder cancer, significantly increasing the
chances of therapeutic success.
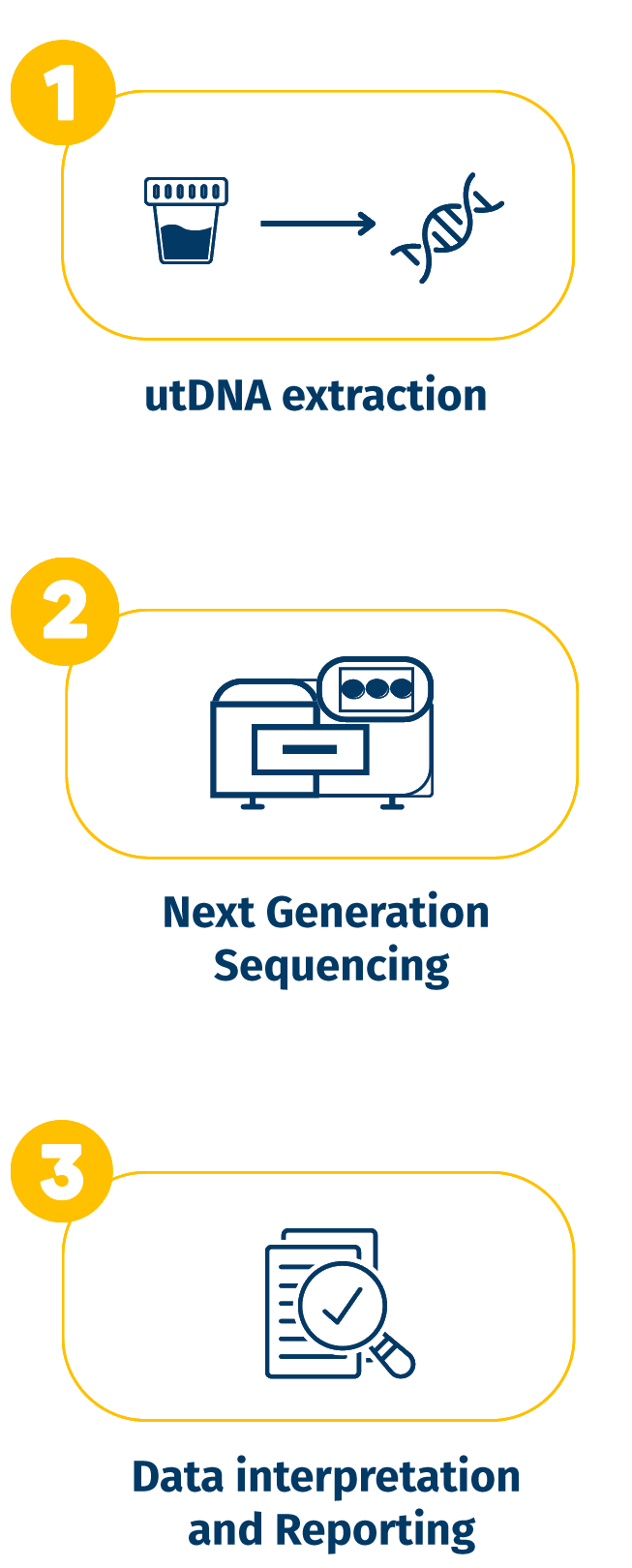
The test uses the latest technological innovations developed for liquid biopsy. Thanks to Next
Generation Sequencing (NGS) sequencing technology, it is now possible to effectively identify somatic
mutations even in the presence of small quantities of tumor cells.
UROADVANCE is able to detect somatic mutations present in minimal percentages (up to 0.5% of
Mutant Allele Frequency - MAF).
Providing sensitivity >90%â´-â¶, the UROADVANCE test allows urologists to make an informed and
confident decision to determine which patients may need further investigation and which may not.
AIMS OF THE TEST

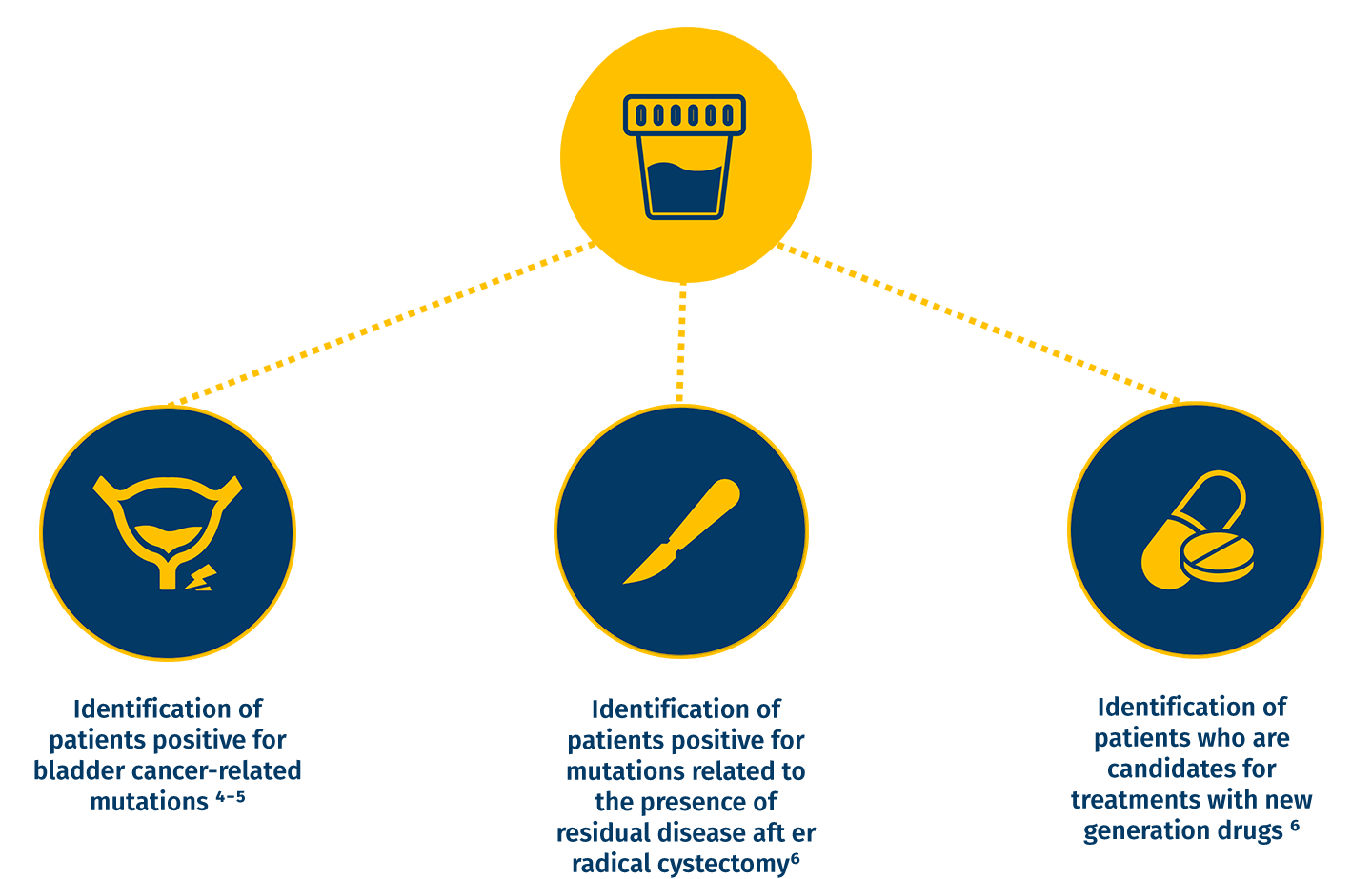

Used throughout the entire bladder cancer treatment pathway, including patient monitoring, surveillance for recurrence and minimal residual disease (MRD), as well as haematuria triage â´â»â¶
4. Ward DG, et al. (BJUI, 2019)
5. Ward DG, et al. (EurUrolOncol. 2023)
6. Chauhan PS, et al. (PLoS Med. 2021)
INDICATION FOR TESTING


Patients with urinary symptoms and clinical picture suspicious for bladder cancer.

Patients undergoing radical cystectomy who require monitoring for residual disease and recurrence.
Patients who need to identify eligibility for targeted therapies and new generation anticancer drugs.
GENES AND MUTATIONS SCREENED


Urinary Tumor DNA utDNA extracted from urine sample pellets.

22 genes related to bladder cancer.

450+ hotspot mutations related to bladder cancer.
Genes screened: promoterTERT, FGFR3, PIK3CA, TP53, ERCC2, RHOB, ERBB2, HRAS, RXRA, ELF3, CDKN1A, KRAS, KDM6A, AKT1, FBXW7, ERBB3, SF3B1, CTNNB1, BRAF, C3orf70, CREBBP, and NRAS.
THE TESTING PROCESS


CLINICAL VALIDITY DEMONSTRATED BY SCIENTIFIC STUDIES ON LARGE COHORTS OF PATIENTS
A recent study5 has demonstrated the sensitivity of sequencing techniques for the identification of
somatic mutations of urinary tumor DNA (utDNA), useful for detecting the presence of tumors in the
bladder.
The test was validated on a sample of almost 1000 patients, enrolled in 10 highly specialized centers,
simultaneously identified by a new cystoscopic diagnosis of urothelial carcinoma.
Among the hotspot mutations under study, investigated with the UROADVANCE test, 96% of enrolled patients presented on average 2.5 somatic mutations related to urothelial carcinoma regardless of grade and stage.

Healthy patients
do not have
utDNA in their urine7.

UROADVANCE test is effective in the non-invasive detection of bladder cancer in the context of haematuria investigations and non-muscle invasive bladder cancer (NMIBC) surveillance6.
5. Ward DG, et al. (BJUI, 2019)
6. Ward DG, et al. (EurUrolOncol. 2023)
7. Chauhan PS, et al. (PLoS Med. 2021)
HIGH SENSITIVITY AND SPECIFICITY IN ALL STAGES OF BLADDER CANCER

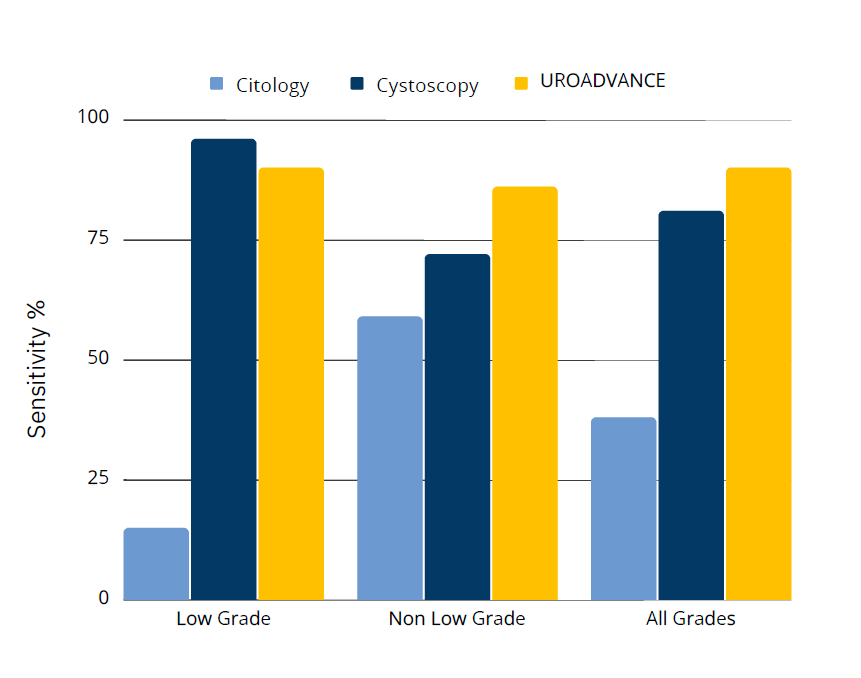
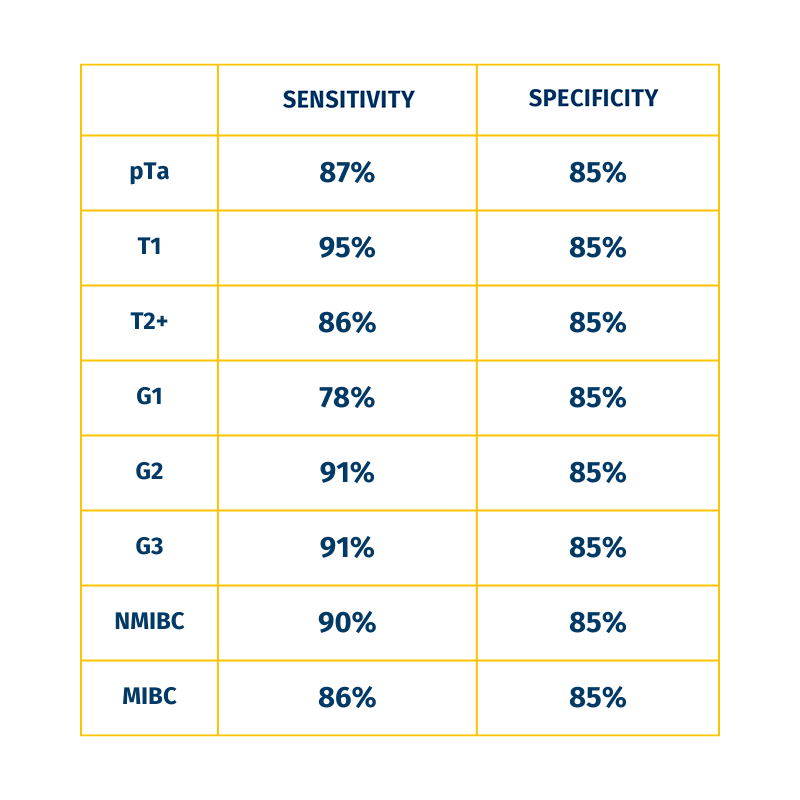



3. Yafi et al. 2015. UrolOncol 2015;33, 66 e25-31.
4. Ward DG, et al. (BJUI, 2019)
5. Ward DG, et al. (EurUrolOncol. 2023)
TEST RESULTS


POSITIVE
This test result indicates that one or more
pathogenic mutations have been detected in the targeted genes screened.

NEGATIVE
This test result indicates that no
pathogenic mutations have been detected in the targeted genes screened.
The identification of a somatic mutation can have different implications, depending on the variant detected. Our geneticist, during genetic counseling, will explain in detail the meaning of the test result.
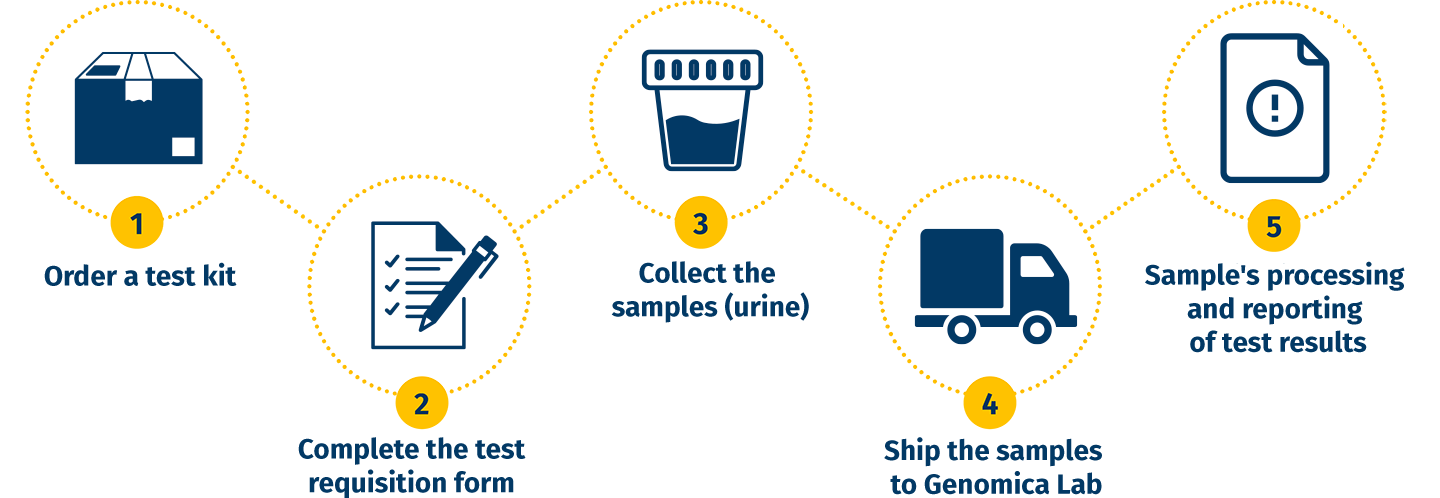
5 EASY STEPS TO GET TESTED




WHY CHOOSE

ADVANCED MOLECULAR DIAGNOSTICS SOLUTIONS USING STATE-OF-THE ART TECHNOLOGIES

GENOMICA is recognized as one of the most advanced molecular diagnostics laboratory in Europe, both for the state-of-the- art instruments and technologies, as well as for its high quality standards. With a comprehensive portfolio of over 10.000 genetic tests, GENOMICA is able to satisfy increasingly specialised requests in the field of molecular genetics, providing physicians and their patients with innovative and highly specialised diagnostic solutions for any clinical need.
Worldwide genetic testing provider
Laboratories with groundbreaking technologies and high quality standards
Dedicated R&D team
Professionals with 20+ years experience in the field of genetics and molecular diagnostics
Personalized genetic counseling with genetic counselors experts in discussing genetic test results and familial risks
Fast TAT 15 days
WHY CHOOSE

ADVANCED MOLECULAR DIAGNOSTICS SOLUTIONS USING STATE-OF-THE ART TECHNOLOGIES

GENOMICA is recognized as one of the most advanced molecular diagnostics laboratory in Europe, both for the state-of-the- art instruments and technologies, as well as for its high quality standards. With a comprehensive portfolio of over 10.000 genetic tests, GENOMICA is able to satisfy increasingly specialised requests in the field of molecular genetics, providing physicians and their patients with innovative and highly specialised diagnostic solutions for any clinical need.
Worldwide genetic testing provider
Laboratories with groundbreaking technologies and high quality standards
Dedicated R&D team
Professionals with 20+ years experience in the field of genetics and molecular diagnostics
Personalized genetic counseling with genetic counselors experts in discussing genetic test results and familial risks
Fast TAT 15 days
Information request for UROADVANCE test
Fill out this form for a free consultation. One of our professionals will contact you, free of charge and without obligation, to provide you with all the information you need.


 Italiano
Italiano English
English